Dissolution Testing USP 1/2/5/6
Dissolution tests are used in the pharmaceutical industry in order to characterize the dissolution properties of the active drug, the active drug's release, as well as the dissolution from a dosage formulation. Different dissolution test...
Read more
Dissolution Testing USP 4
The flow-through dissolution method (USP 4 / EP Flow-through cell) offers complete flexibility on media volumes and allows repeatable positioning of virtually all dosage forms: powders, suspensions, suppositories, APIs, lipophilic forms,...
Read more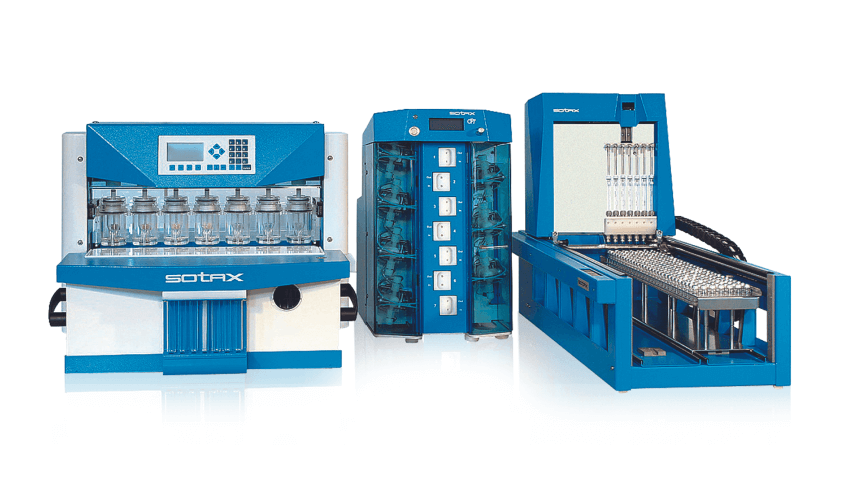
Physical Testing Instruments
SOTAX offers solutions for testing the physical properties of tablets, capsules and other solid dosage forms: reliable, precise and easy-to-operate tablet hardness testers and disintegration testers, robust devices for friability testing...
Read more
Automated Sample Preparation
Volumetric preparation of solid or liquid oral dosage forms, API, creams and pastes requires accurate execution of multiple laborious steps. Automated sample preparation boosts laboratory productivity by minimizing resource allocation fo...
Read more
JetX™ MultiFlow

