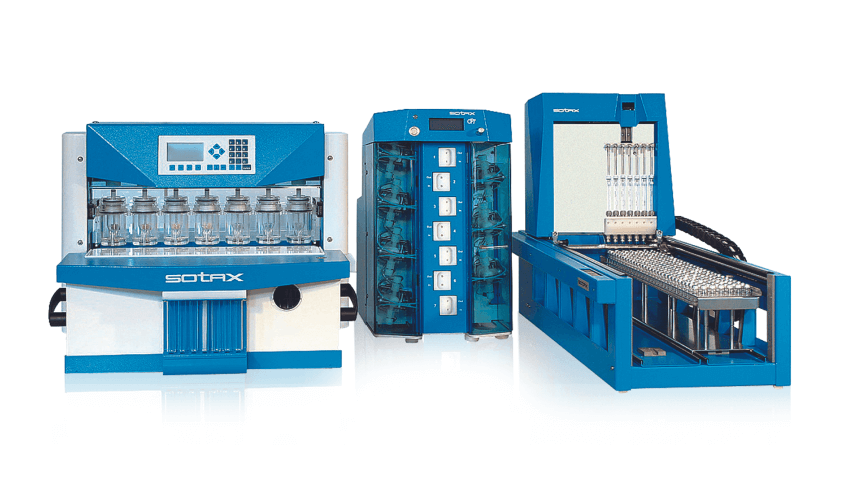
CE 7smart UV On-/Offline
CE 7smart UV On-/Offline is a USP 4 compliant flow-through cell dissolution testing system for online UV-Vis analysis and automated, offline collection of filtered samples. In the open loop setup, offline collection and online ...
Read more

CE 7smart UV Online
The SOTAX's CE 7smart UV Online flow-through cell dissolution testing system has integrated UV-Vis analysis of samples in real-time. These online UV-Vis measurements can be done both in open or closed loop configuration. For on...
Read more

CE 7smart Offline
CE 7smart Offline is a flow-through cell dissolution testing system available in open or closed loop configuration for automated sample and fraction collection. Both configurations can be programmed via the
firmware to collect...
Read more